Ensuring Product Integrity and Long-Term Brand Reliability by TIBD
In today’s cosmetics industry, where innovation and credibility define market leadership, having a well-designed formulation alone is no longer sufficient. Successful brands must be able to maintain consistent product quality and consumer experience throughout the entire product lifecycle. A critical factor in achieving this consistency is the compatibility between the cosmetic formulation and its packaging system.
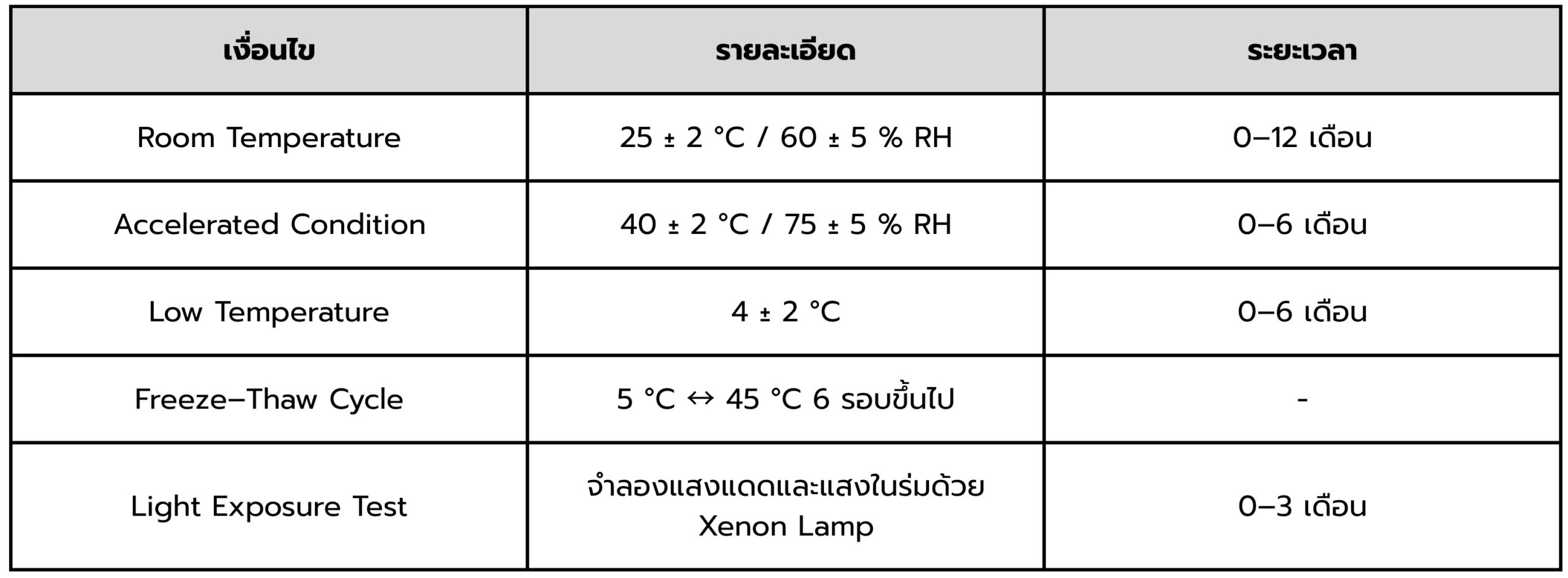
At TIBD, we consider Packaging Compatibility Testing not merely a laboratory procedure, but a strategic technology management tool that enables brands to understand product behavior in depth and apply scientific data to informed business decision-making.